本文内容已经过同行评议,以优先出版方式在线发表,可作为有效引用数据。由于优先发表的内容尚未完成规范的编校流程,《中华外科杂志》不保证其数据与正式版内容的完全一致。 【引用本文】喻彦熹,吴忠均,唐伟,等.肝内胆管癌国际临床实践指南和共识的诊疗建议比较[J].中华外科杂志,2023,61(4):297-304.
肝内胆管癌(ICC)是人类第二常见的肝脏恶性肿瘤,在过去的几十年里,其发病率在全球范围内逐渐上升。根治性手术切除是ICC患者首选的可能根治的治疗手段。但ICC起病隐匿,侵袭性较高,多数患者确诊时已失去手术机会。另外,近年来以靶向治疗和免疫检查点抑制剂为代表的免疫治疗的迅速发展,有望为中晚期ICC患者提供更有效的治疗。目前,国内外各指南在ICC术前胆道引流、肝切除术范围、根治性切除的定义、切缘宽度、常规淋巴结清扫、术后复发、辅助治疗等方面存在不同程度的差异。本文检索了2012—2022年国内外发布的12篇针对或涵盖ICC临床诊疗实践的指南或共识,重点整理和比较了当前各指南在临床管理上的观点,旨在为临床工作提供数据参考以辅助临床决策。
肝内胆管癌(intrahepatic cholangiocarcinoma,ICC)起源于肝内胆管上皮细胞,是人类第二常见的肝脏恶性肿瘤(占原发性肝癌的10%~15%),发病率仅次于肝细胞癌(hepatocellalar carcinoma,HCC),并呈现明显的地区、种族、性别差异[1]。ICC的发生多与胆管内的慢性炎症和长期胆汁淤积有关,但绝大多数病例并不存在已知或可疑的危险因素[1],这为ICC的常规筛查和临床管理提出了挑战。
目前,单独针对ICC的临床实践指南相对较少,常包含于胆管癌的临床指南中。我们通过检索中国生物医学文献服务系统、中国知网CNKI数据库、MEDLINE等文献数据库,收集了2012—2022年全球范围内发布的12篇针对或涵盖ICC临床诊疗信息的共识、指南,其中4份来自中国、4份来自欧洲、2份来自美国、2份来自日本(表1),重点比较各指南(共识)在ICC临床管理观点上的异同,旨在为临床工作提供数据参考以辅助临床决策。
表1 2012—2022年国内外发表的肝内胆管癌相关指南和共识一览表a

一、诊断
二、临床分期
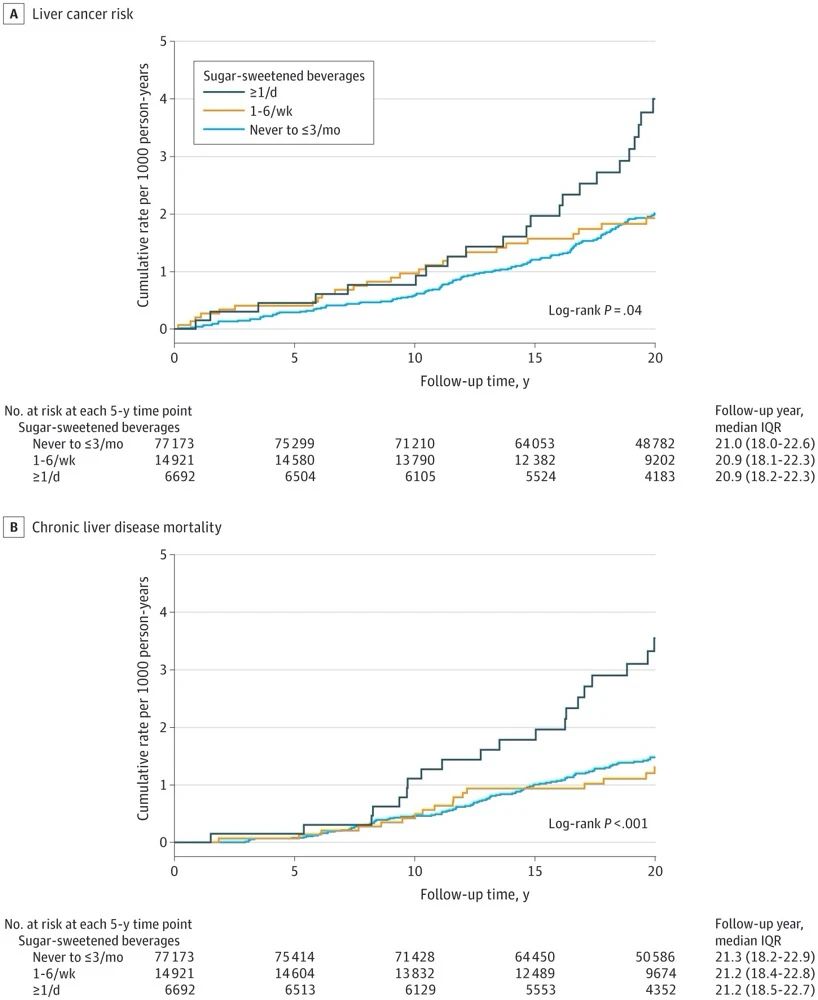
三、治疗方法(图1)

四、小结
参考文献
[1]KhanSA,TavolariS,BrandiG. Cholangiocarcinoma:epidemiology and risk factors[J].Liver Int,2019,39 Suppl 1∶19-31. DOI: 10.1111/liv.14095.
[2]KuboS,ShinkawaH,AsaokaY,et al. Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma[J].Liver Cancer,2022,11(4):290-314. DOI: 10.1159/000522403.
[3]中国抗癌协会肝癌专业委员会胆管癌协作组. 原发性肝癌诊疗指南之肝内胆管癌诊疗中国专家共识(2022版)[J].中华消化外科杂志,2022,21(10):1269-1301. DOI: 10.3760/cma.j.cn115610-20220829-00476.
[4]BensonAB,D′AngelicaMI,AngelicaMI,et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology[J].J Natl Compr Canc Netw,2021,19(5):541-565. DOI: 10.6004/jnccn.2021.0022.
[5]BanalesJM,MarinJ,LamarcaA,et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management[J].Nat Rev Gastroenterol Hepatol,2020,17(9):557-588. DOI: 10.1038/s41575-020-0310-z.
[6]BridgewaterJ,GallePR,KhanSA,et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma[J].J Hepatol,2014,60(6):1268-1289. DOI: 10.1016/j.jhep.2014.01.021.
[7]KhanSA,DavidsonBR,GoldinRD,et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update[J].Gut,2012,61(12):1657-1669. DOI: 10.1136/gutjnl-2011-301748.
[8]Cholangiocarcinoma Working Group.Italian Clinical Practice Guidelines on Cholangiocarcinoma-Part Ⅰ: Classification, diagnosis and staging[J].Dig Liver Dis,2020,52(11):1282-1293. DOI: 10.1016/j.dld.2020.06.045.
[9]WeberSM,RiberoD,O′ReillyEM,et al. Intrahepatic cholangiocarcinoma: expert consensus statement[J].HPB (Oxford),2015,17(8):669-680. DOI: 10.1111/hpb.12441.
[10]NaginoM,HiranoS,YoshitomiH,et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition[J].J Hepatobiliary Pancreat Sci,2021,28(1):26-54. DOI: 10.1002/jhbp.870.
[11]ShenZT,ZhouH,LiAM,et al. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma[J].Oncotarget,2017,8(55):93541-93550. DOI: 10.18632/oncotarget.19972.
[12]ChongYS,KimYK,LeeMW,et al. Differentiating mass-forming intrahepatic cholangiocarcinoma from atypical hepatocellular carcinoma using gadoxetic acid-enhanced MRI[J].Clin Radiol,2012,67(8):766-773. DOI: 10.1016/j.crad.2012.01.004.
[13]胆道肿瘤专家委员会. CSCO胆道系统肿瘤诊断治疗专家共识(2019年版)[J].临床肿瘤学杂志,2019,24(9):828-838. DOI: 10.3969/j.issn.1009-0460.2019.09.014.
[14]科技部传染病防治重大专项课题"病毒性肝炎相关肝癌外科综合治疗的个体化和新策略研究"专家组. 肝内胆管癌外科治疗中国专家共识(2020版)[J].中华消化外科杂志,2021,20(1):1-15. DOI:10.3760/cma.j.cn115610-20201211-00777.
[15]HyderO,MarquesH,PulitanoC,et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience[J].JAMA Surg,2014,149(5):432-438. DOI: 10.1001/jamasurg.2013.5168.
[16]SakamotoY,KokudoN,MatsuyamaY,et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan[J].Cancer,2016,122(1):61-70. DOI: 10.1002/cncr.29686.
[17]Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma-Part Ⅱ: Treatment[J].Dig Liver Dis,2020,52(12):1430-1442. DOI: 10.1016/j.dld.2020.08.030.
[18]LiDY,ZhangHB,YangN,et al. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series[J].World J Gastroenterol,2013,19(47):9084-9091. DOI: 10.3748/wjg.v19.i47.9084.
[19]国际肝胆胰学会中国分会,中华医学会外科学分会肝脏外科学组. 胆管癌诊断与治疗——外科专家共识[J]. 中国实用外科杂志, 2014, 34(1): 1-5.
[20]LunsfordKE,JavleM,HeyneK,et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series[J].Lancet Gastroenterol Hepatol,2018,3(5):337-348. DOI: 10.1016/S2468-1253(18)30045-1.
[21]PrimroseJN,FoxRP,PalmerDH,et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study[J].Lancet Oncol,2019,20(5):663-673. DOI: 10.1016/S1470-2045(18)30915-X.
[22]EbataT,HiranoS,KonishiM,et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer[J].Br J Surg,2018,105(3):192-202. DOI: 10.1002/bjs.10776.
[23]EdelineJ,BenabdelghaniM,BertautA,et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase Ⅲ study[J].J Clin Oncol,2019,37(8):658-667. DOI: 10.1200/JCO.18.00050.
[24]ValleJ,WasanH,PalmerDH,et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J].N Engl J Med,2010,362(14):1273-1281. DOI: 10.1056/NEJMoa0908721.
[25]IokaT,KanaiM,KobayashiS,et al. Randomized phase Ⅲ study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA)[J].J Hepatobiliary Pancreat Sci,2023,30(1):102-110. DOI: 10.1002/jhbp.1219.
[26]SiaD,LosicB,MoeiniA,et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma[J].Nat Commun,2015, 6:6087. DOI: 10.1038/ncomms7087.
[27]PostowMA,SidlowR,HellmannMD. Immune-related adverse events associated with immune checkpoint blockade[J].N Engl J Med,2018,378(2):158-168. DOI: 10.1056/NEJMra1703481.